NON-COMMERCIAL, RANDOMIZED, MULTICENTER, PROSPECTIVE, INTERVENTIONAL CLINICAL TRIAL
IREC Study
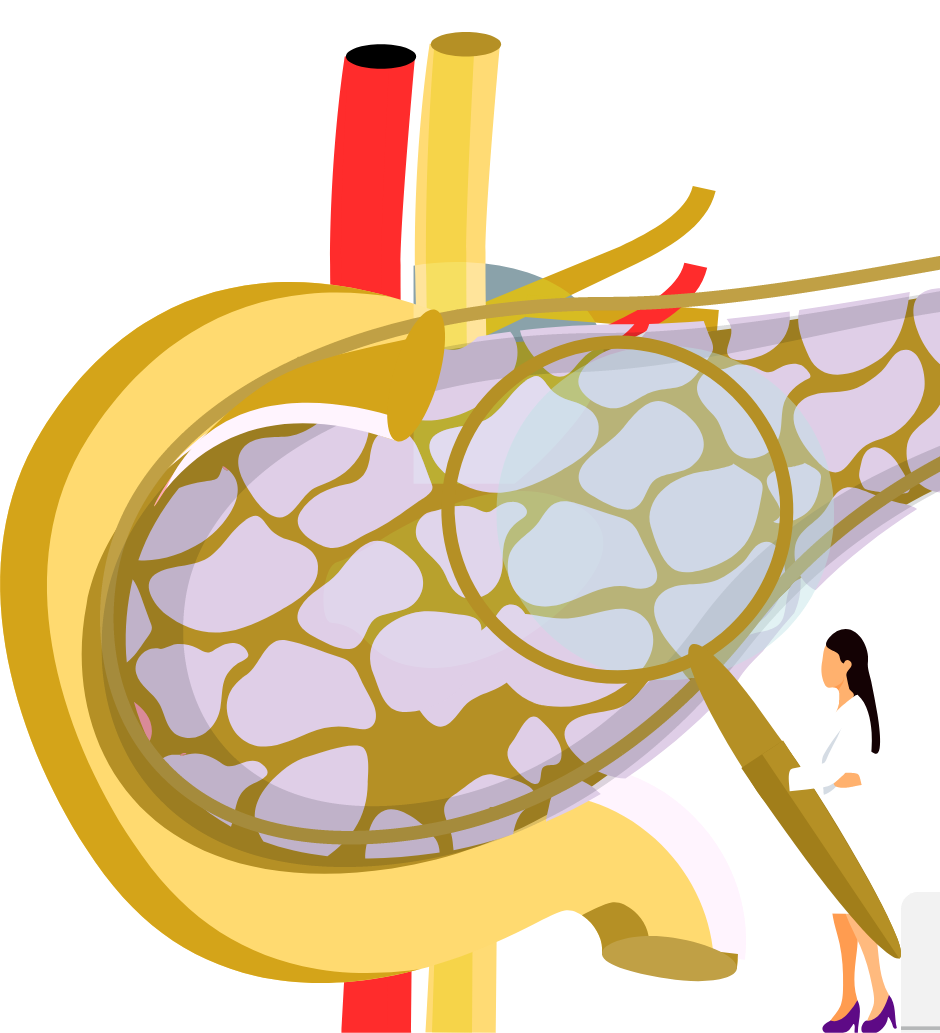
Impact of irreversible electroporation with calcium chloride, electrochemotherapy and electroporation (IRE-CaCl2, ECT and IRE) on quality of life and progression-free survival in patients with pancreatic cancer (IREC)
Dear Sir/Madam,
We invite you to participate in a phase III interventional clinical trial, acronym IREC, aimed at developing a new form of pancreatic cancer treatment based on irreversible electroporation, calcium electroporation, and electrochemotherapy.
The study will assess the impact of the therapy on the quality of life and progression-free survival of patients.
zIf you need assistance,
please contact:
BioStat Sp. z o.o.
Kowalczyka 17, 44-206 Rybnik
tel: 666-069-834
email: [email protected]

Login
im. Piastów Śląskich we Wrocławiu
wyb. Ludwika Pasteura 1,
50-367 Wrocław